What Type of Reaction Is Bacl2 + Na2so4
Chemistry questions and answers. Na2SO4BaCl2BaSO42NaCl which types of reaction.

Crystal Structure Of Metal Crystals Crystal Structure Bravais Lattice
The barium sulfate is formed as a precipitate.

. Types of chemical reactions. A Displacement b Precipitation c Combination d Double displacement. Add your answer and earn points.
As a result of the above-given reaction a white colour precipitate is formed due to the formation of barium sulphate. Covalent compounds are generally poor conductors of electricity. What are some examples of double.
First you have to use concentrated sulfuric acid. The precipitation of barium sulfate - a reaction used in gravimetry. The reactants changes into ions when dissolved in water and there is an exchange of ions in solution.
B BaCl2 is a liquid and Na2SO4 is a gas. BaCl2 aq Na2SO4 aq BaSO4 s 2NaCl aq A combination B decomposition C double displacement D single displacement Which of the following reactions is a double displacement reaction. Or jo equal ka symbol hai matlbe hai arrow.
1 mole of sulfuric acid reacts with 2 moles of sodium hydroxide to produce 1 mole of sodium sulfate and 2 moles of water. By Stoichiometry of the reaction. When barium chloride BaCl 2 and sodium sulfate Na 2 SO 4 which leads to the formation of sodium chloride and barium sulphate.
Eni Doku s answer is correct though it needs to be balanced and the reaction is. BaCl2 Na2SO4 BaSO4 s 2NaCl aq Barium sulfate is a stable white precipitate. C BaCl2 is a solid and Na2SO4 is in aqueous solution.
BaCl2 aq Na2SO4 aq BaSO4 s 2NaCl. Second the reaction is done at elevated temperature. Heat applied to the reaction mixture aids to.
Ayyash007 ayyash007 11042020 Chemistry Secondary School answered What type of reaction is this aNa2So4Bacl2 gives Bacl22Nacl 1 See answer ayyash007 is waiting for your help. Decomposition single replacement double replacement combination Using the balanced chemical equation determine how many moles of NaCl will be produced if 0169 mol BaCl2 is allowed to react with an excess of Na2SO4. Is BaCl2 H2SO4 BaSO4 HCl balanced.
The reaction takes place in a boiling solution. What type of reaction is BaCl2 plus Na2SO4 equals 2 NaCl plus BaSO4. The reaction between Na2SO4 BaCl2 is an endothermic reaction.
In this video we determine the type of chemical reaction for the equation BaCl2 Na2SO4 BaSO4 NaCl Barium chloride Sodium sulfateSince we have a tw. A HCI g H2 g Cl 9 B HCI aq NaOH aq - HO 1. However there are some technical considerations.
For the given chemical equation the balanced reaction follows. D Both reactants are in aqueous solution. What happens when BaCl2 reacts with Na2SO4.
In the reaction BaCl2 aq Na2SO4 aq BaSO4 s 2NaCl aq what phases are the reactants in before the reaction. The chemical equation BaCl2 Na2SO4 to BaSO4 2NaCl represents the following type of chemical reaction ADisplacement BCombination CDecomposition DDoubledisplacement. BaCl2 Na2SO4 BaSO4 2 NaCl Balanced equation Chemical Equations online.
BaCl2 aq Na2SO4 aq BaSO4 s 2NaCl aq Above reaction involves which type of reaction. Chemistry questions and answers. BaCl2aqNa2SO4aq BaSO4sNaClaq Which type of reaction is this.
Click here to find more information about this equation. A Both reactants are gases. X and Y are two homologous carbon compounds whose boiling point are given in the table 1 which molecule will have higher molecular mass.
Advanced Search with assistance of Google Search Engine. 2 NaCl H2SO4 Na2SO4 2 HCl. Chemical Reactions and Equations.
The chemical equation BaCl2 Na2SO4 Ba Question The chemical equation B a C l 2 N a 2 S O 4 B a S O 4 2 N a C l represents following type of chemical reaction. In this experiment the mixture of two solutions sodium sulfate Na2SO4 and barium chloride BaCl2 forms a precipitate. Sample question 2 Higher.
Question 42 of 45 What type of reaction is represented by the following equation. Hence we change the coefficients of the compounds in the given chemical reaction. A precipitate is a solid formed during a reaction between two aqueous compounds.
What type of reaction is this aNa2So4Bacl2 gives Bacl22Nacl Get the answers you need now. BaCl2 aq Na2SO4 aq BaSO4 s 2NaCl aq A combination B decomposition C double displacement D single displacement. What type of reaction is used to prepare barium sulphate.
What happens when barium chloride reacts with Aluminium sulphate. When barium chloride combines with sodium sulphate in the form of their aqueous solutions a white precipitate of barium sulphate is formed which is insoluble in water. We have been working with Google to develop an advanced search with results filted with chemistry topic only.
What type of reaction is represented by the following equation. This is a double replacement reaction which forms barium sulfate and salt NaCl when the sodium ions in sodium sulfate. In this video well balance the equation BaCl2 Na2SO4 BaSO4 NaCl and provide the correct coefficients for each compoundAll of the compounds in this re.
Sunphate SO4 in Na2SO4 reacts with barium in BaCl2 to create a white precipitate of barium sunphate. Barium sulfate is prepared by reacting barium chloride with sodium sulfate. What type of reaction is BaCl2 na2 so4 BaSO4 2NaCl.
These reactions are ionic in nature.
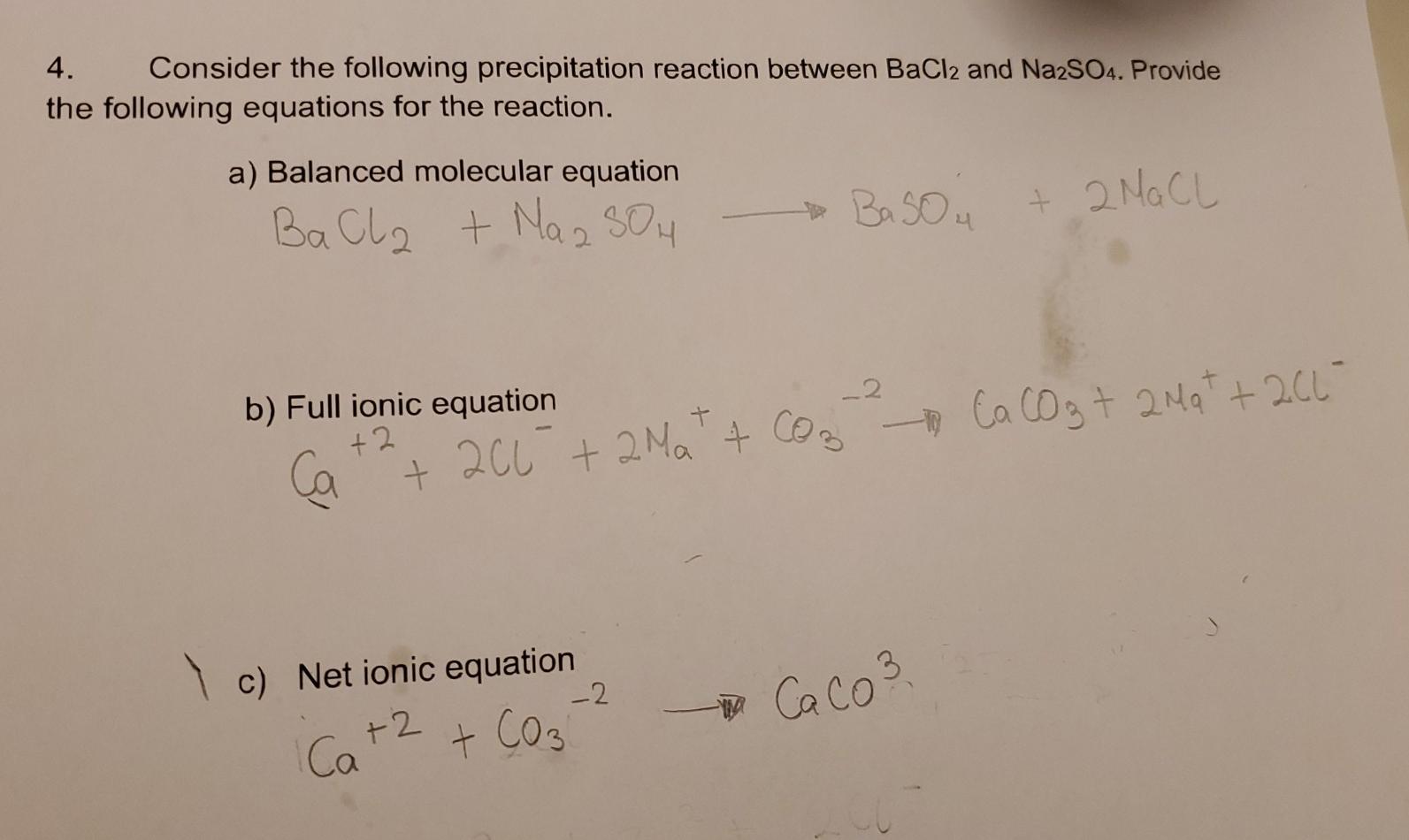
Solved 4 Consider The Following Precipitation Reaction Chegg Com

Bacl2 Na2so4 Baso4 Nacl Balanced Chemical Equation

Ncert Solutions For Class 10 Science Chapter 1 Chemical Reactions Equations Chemical Reactions Equations Science

Net Ionic Equations Ap Chem Equations Chemistry Class

Type Of Reaction For Bacl2 Na2so4 Baso4 Nacl Youtube

How To Write The Net Ionic Equation For Bacl2 Na2so4 Baso4 Nacl Youtube

Double Displacement Sodium Sulfate And Barium Chloride Chemistry Classroom Voss Bottle Core Collection

Barium Chloride And Sodium Sulfate React According To The Following Equation Bacl2 Na2so4 Baso4 2naci Answer Homeworklib

Bacl2 Aq Na2so4 Aq Baso4 S 2nacl Aq Above Reaction Involves Which Type Of Reaction A Displacement B Precipitation C Combination D Double Displacement
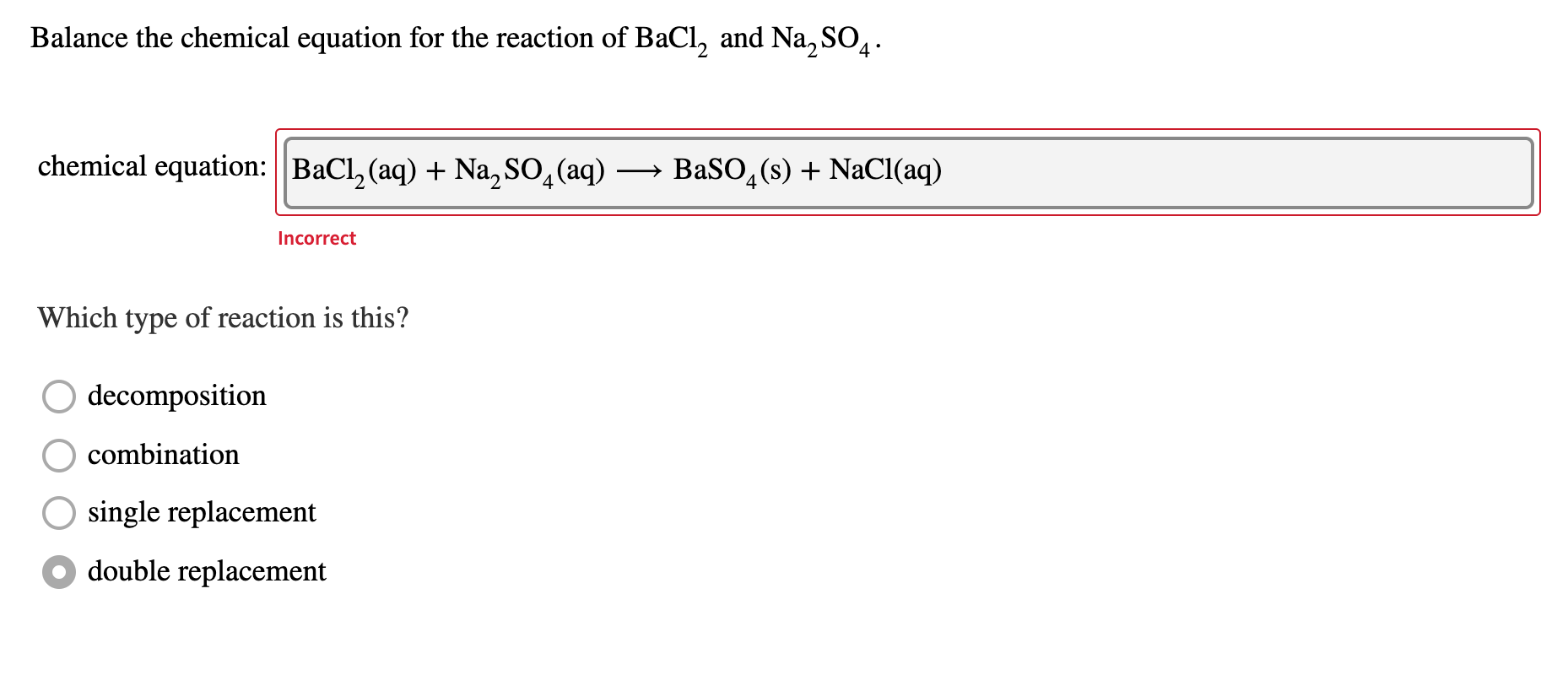
Solved Balance The Chemical Equation For The Reaction Of Chegg Com

Double Displacement Sodium Sulfate And Barium Chloride Chemistry Classroom Voss Bottle Core Collection
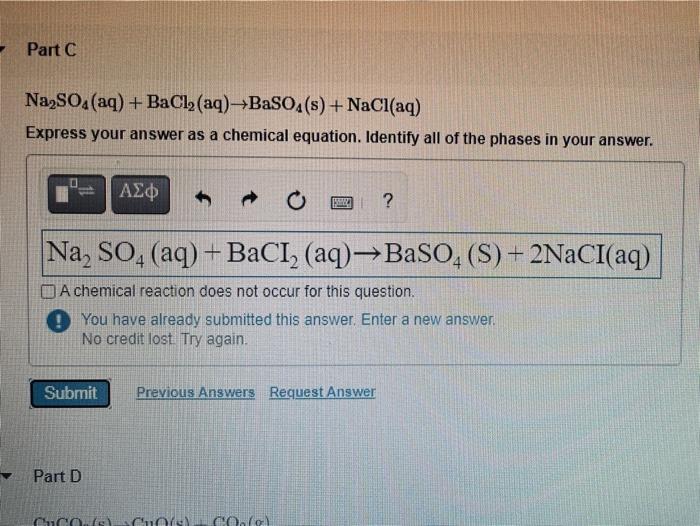
Solved Part C Na2so4 Aq Bacl2 Aq Baso4 S Nacl Aq Chegg Com
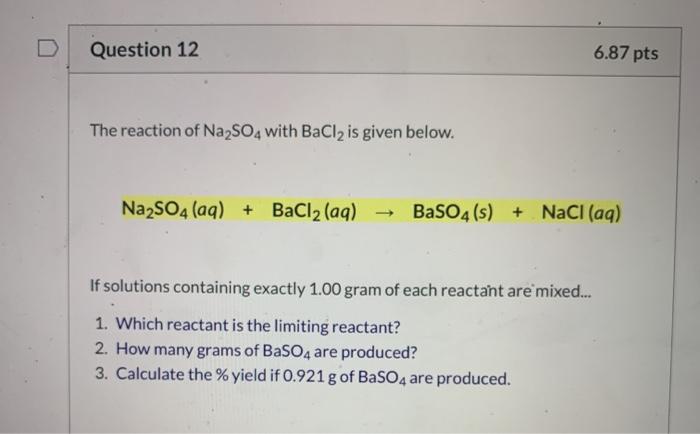
Solved Question 12 6 87 Pts The Reaction Of Na2so4 With Chegg Com

Balancing Nuclear Equations Worksheet Answers Beautiful Balancing Nuclear Equations Worksheet Chemistry Worksheets Chemical Equation Word Problem Worksheets

Bacl2 Na2so4 Baso4 Nacl Balance The Chemical Equation Bacl2 Na2so4 Baso4 Nacl Youtube

How To Balance Bacl2 Na2so4 Baso4 Nacl Barium Chloride Sodium Sulfate Youtube

10th Science Practical Based Questions In Chemical Reactions Equations Chemical Reactions Equations Chemical Equation
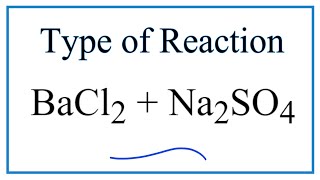
Comments
Post a Comment